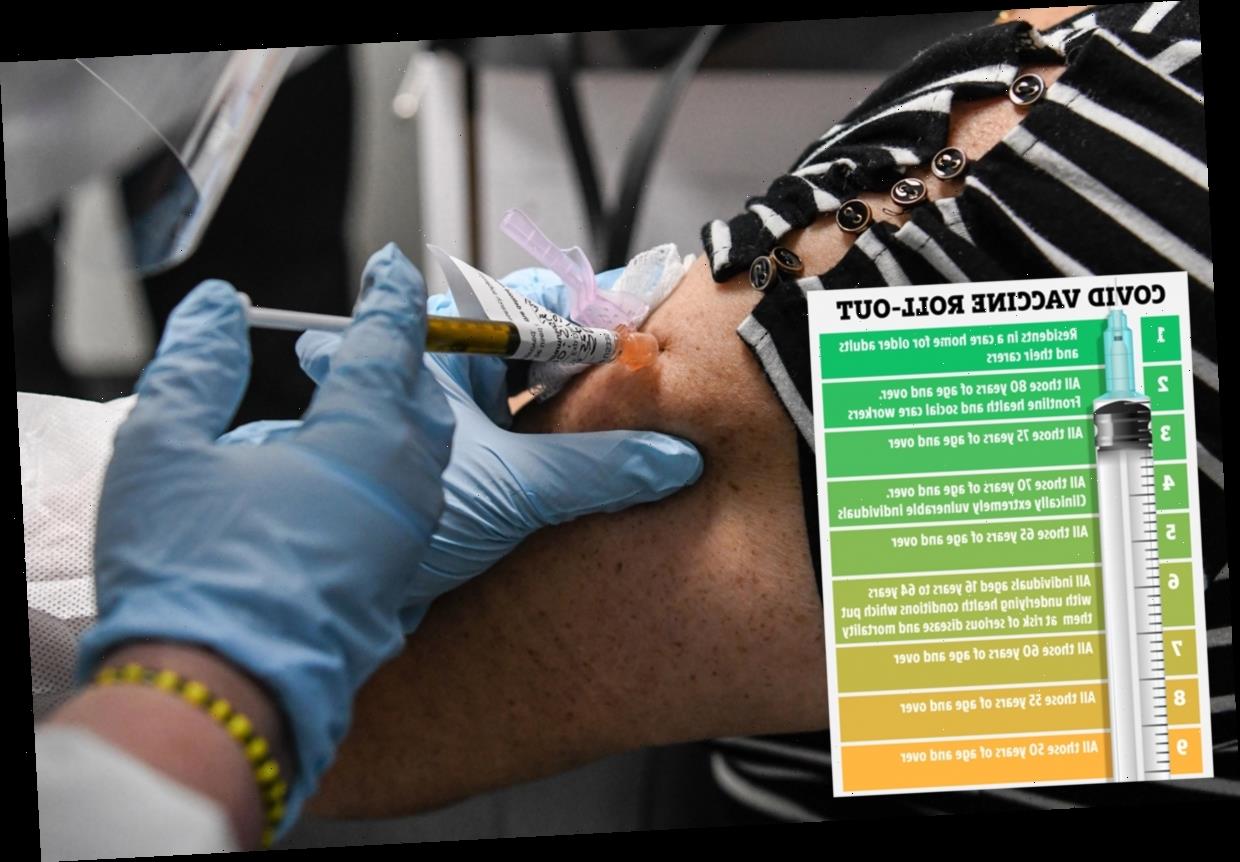
CARE home residents and NHS staff will be first in line for the Pfizer vaccine, experts have confirmed.
People aged 16 years to 64 years with underlying health conditions which put them at higher risk will be sixth in line for the jab after older age groups.
⚠️ Read our coronavirus live blog for the latest news & updates
It comes as health regulators revealed that the Pfizer/BioNTech jab, which is 95 per cent effective has been given the green light to be rolled out across the UK.
Regulators this morning addressed the nation as the NHS gets set to start it's mass vaccination programme.
The chief executive of the Medicines and Healthcare products Regulatory Agency (MHRA), Dr June Raine lead the briefing.
Professor Sir Munir Pirmohamed (Chair of Commission on Human Medicine Expert Working Group) and Prof Wei Shen Lim, chair of the Joint Committee on Vaccination and Immunisation (JCVI) were also in attendance.
Care home residents, health and care staff, the elderly and the extremely vulnerable will be among the 800,000 to get the jab in the first wave next week, it was confirmed.
After the most vulnerable people have received the vaccine the over 60s will receive the jab.
This will be followed by the over 55s and 50s before the rest of the population is able to be vaccinated.
Speaking at the Downing Street briefing Dr Raine said top standards had been maintained during production and testing of the vaccine.
"That doesn't mean that any corners have been cut, none at all."
She added that the public would be invited to take part in an active monitoring programme which would see people receive letters to join.
These, Dr Raine said, would be sent out on a random basis.
WHO ARE THE AT-RISK GROUPS?
The elderly, NHS staff and other at-risk groups will take priority when it comes to a Covid jab.
People over 18 with the following conditions are considered to be in 'at-risk' groups:
- chronic respiratory disease like severe asthma, COPD and cystic fibrosis
- chronic heart disease and vascular disease
- chronic kidney disease
- chronic liver disease
- chronic neurological disease like cerebral palsy, Parkinson's, dementia, motor neurone disease
- type 1 diabetes
- type 2 diabetes requiring insulin or oral medication, diet-controlled diabetes
- immunosuppression – those undergoing chemo, transplant patients, people taking certain drugs that suppress the immune system
- asplenia or dysfunction of the spleen
- morbid obesity – those with a body mass index of over 40
- severe mental illness
- adult carers
- close relatives or carers of immunocompromised adults
- younger adults in long-stay nursing homes and residential care settings
GREEN LIGHT
Dr Raine said experts had worked "round the clock, carefully, methodically poring over tables and analyses and graphs on every single piece of data".
More than 1,000 pages of data had been examined, she said.
A report published by the government stated that "all evidence indicates that the best option for preventing morbidity and mortality in the initial phase of the programme is to directly protect persons most at risk of morbidity and mortality".
The experts today stressed that the JCVI list is paramount to distribution and said it would not matter what tier a person was in.
Prof Shen Lim said that the JCVI had considered the safety data on the jab.
"We’re pleased to say that it supports vaccinating those most at-risk of death from Covid-19 – starting with older people in care homes and those aged 80 years and above.
“This priority reflects the available data on those most at-risk of serious disease and death from Covid-19 infection.
"Our advice will be updated depending on the safety and characteristics of other vaccines, once available.”
Just days ago, it was announced that the drug was set to get the green light for use – and medics were told to prepare for approval in early December.
And it's now been announced that almost a million vaccine doses will be available from next week, with "several millions" more coming throughout December.
The first shipments will arrive as early as today, although the bulk of the roll-out will take place in the new year.
'BIG STEP FORWARD'
Dr Mary Ramsay, Head of Immunisations at Public Health England (PHE, said the recommendations from the JCVI and MHRA provide confidence that the Pfizer/BioNTech vaccine has met the "very high standards needed to roll out the vaccine".
She added: "This is a big step forward in tackling the virus.
“This means it can be delivered to those most at-risk, to help prevent as many deaths from Covid-19 as possible.
"Once deployed, PHE will work alongside the MHRA to keep the safety and efficacy of the vaccine under constant review.”
AGE MATTERS
The JCVI states that age is the biggest risk when it comes to the coronavirus.
A report published by the group states that models show that the vaccine is safe on older adults.
The report states: "Data also indicate that the absolute risk of mortality is
higher in those over 65 years than that seen in the majority of youngeradults with an underlying health condition."
The report also notes that care home residents have been "disproportionally affected" by Covid-19.
This, the report states, is due to the fact that they are at a higher risk of being exposed to infection.
"The Committee’s advice is that this group should be the highest priority for vaccination. Vaccination of residents and staff at the same time is considered to be a highly efficient strategy within a mass vaccination programme with the greatest potential impact", the report adds.
The UK regulator was formally asked by Health Secretary Matt Hancock to check the Pfizer vaccine and approve it.
He said today: "From Easter onwards, things are going to be better and we’re going to have a summer next year that everybody can enjoy."
Mr Hancock tweeted earlier this morning: "Help is on its way.
"The MHRA has formally authorised the Pfizer/BioNTech vaccine for Covid-19.
"The NHS stands ready to start vaccinating early next week.
"The UK is the first country in the world to have a clinically approved vaccine for supply."
Hospitals are now preparing their staff to start receiving the vaccine from Monday. An army of helpers will then issue the jab to those most in need.
It has to be stored at -70C and can only be thawed in batches of 1,000 before immunisation.
Source: Read Full Article