Scientists world over have been working day and night to find an end for the deadly coronavirus, which has killed more than 26,000 people in the UK. A drug called Remdesivir is among a handful which scientists and the World Health Organization consider to be promising.
What is Remdesivir?
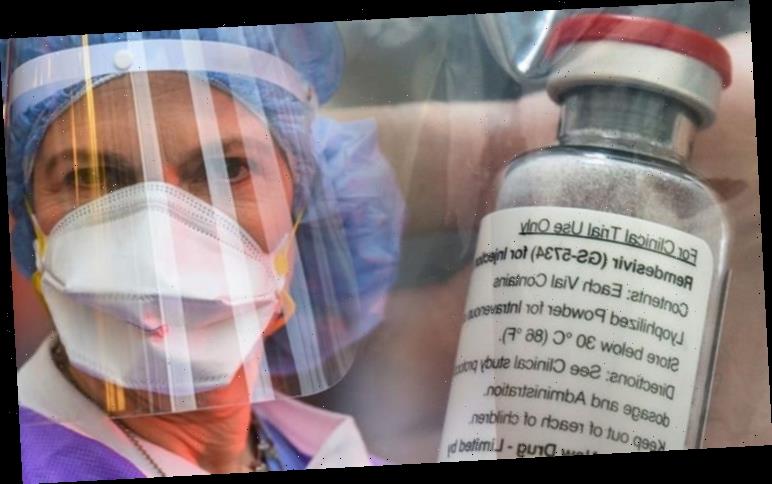
Remdesivir is an experimental antiviral drug produced by the US pharmaceutical company Gilead.
The drug was initially used as a potential treatment for the Ebola virus, which sparked a crisis in Africa several years ago.
The drug has been rapidly pushed through clinical trials after showing promising results in tests and was then rolled out more widely in affected areas of Africa.
READ MORE
-
Coronavirus and newborns: Can mothers give their newborns COVID-19?
The drug is administered via daily infusion for about ten days, although scientists are also working on producing it to be administered by an inhaler.
Currently, Remdesivir has not been approved by health authorities around the world and supply is low.
However, Gilead has indicated they are planning to produce a million units by the end of this year.
Despite not being fully approved by the FDA in America, it is still being used in clinical trials, and many countries have been scrambling for access to the drug for use in their own studies.
Does Remdesivir work?
In a trial of more than 1,000 patients, those given Remdesivir improved after an average of 11 days, compared with an average of 15 days for those not given the treatment.
No statistically significant improvement in survival rate between the two groups was found.
While the drug hasn’t been given the green light to be used widely in hospitals, it is being used at an Australian hospital in New South Wales and will be used in a further five currently undisclosed medical centres.
DON’T MISS
How long does coronavirus live on clothes?
Could over-50s be in lockdown LONGER than everyone else?
Coronavirus death toll: Is UK WORSE than rest of Europe?
READ MORE
-
Book a coronavirus test: How to book a test for COVID-19
However, the findings of the study into Remdesivir have not actually been published yet, leading to speculation in the scientific community about whether it actually delivers the results it promises.
The US clinical trial registry also shows that Gilead altered the primary outcome measures of the trial halfway through.
The firm broadened its definition of “recovery” and included patients who were still in hospital and who were at home but requiring oxygen in its group of recovered patients.
Changing the primary outcomes of a study is often viewed by scientists with scepticism in any clinical trial.
This is because it is often done to improve the significance of results after the initial outcomes proved disappointing, otherwise known as “fishing for significance.”
What’s more, nine members of the US panel that establishes treatment guidelines have a financial interest in Gilead.
The news comes as immunologist Dr Anthony Fauci promoted the preliminary findings, inspiring hope in the treatment.
Speaking at the White House on Wednesday, Dr Fauci said: “The data shows that Remdesivir has a clear-cut, significant, positive effect in diminishing the time to recovery.
Expressing genuine excitement at the findings, the usually reserved Dr Fauci confidently added: “What it has proven is that a drug can block this virus.”
The UK has begun its clinical trials for a vaccine at Oxford and Imperial College universities, but there are no approved treatments currently available for the virus.
Source: Read Full Article