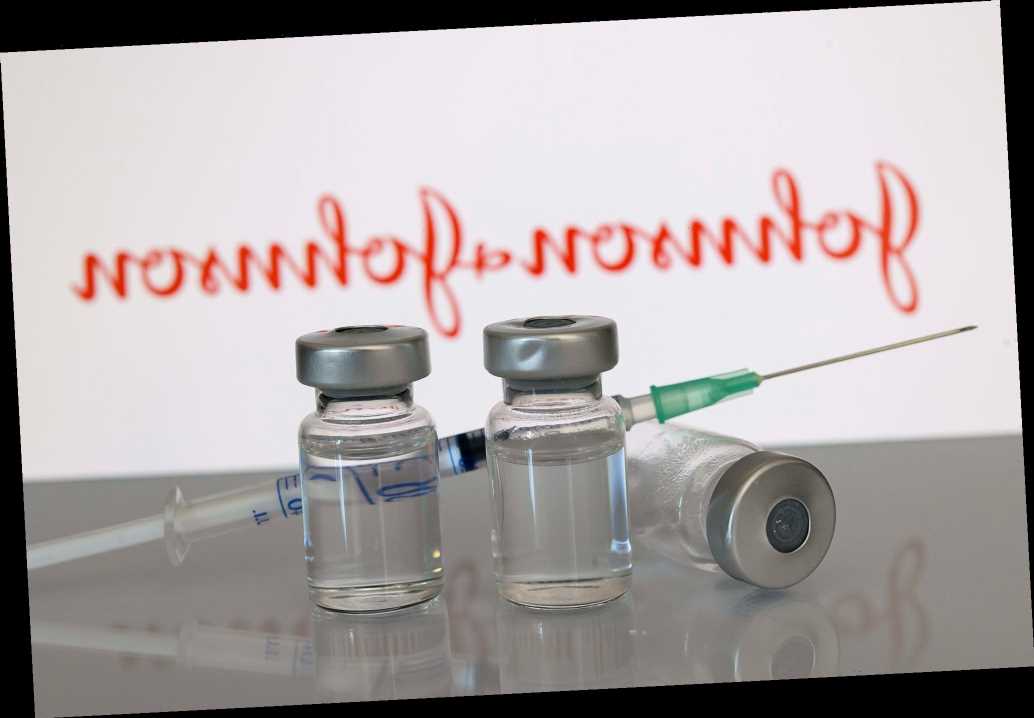
Pharmaceutical competitors Merk & Co and Johnson & Johnson are teaming up in the fight against COVID-19.
According to reports, President Joe Biden is scheduled to announce on Tuesday that Merk will be helping Johnson & Johnson manufacture its COVID-19 vaccine after failing to develop its own, both The Washington Post and The New York Times reported.
"It's a historic partnership," one of Biden's senior administration officials told the Post anonymously. He added that the two competing companies "recognize this is a wartime effort," and praised their sense of "corporate citizenship."
Both outlets reported that the partnership was "brokered" by the White House after officials realized that Johnson & Johnson had fallen behind in vaccine production.
RELATED: 4 Million Doses of Johnson & Johnson COVID Vaccine Are on the Way, Will Arrive as Early as Tuesday
Merk has an extensive history of vaccine production. It is the only supplier of the combination vaccine for children that protects against measles, mumps and rubella in the United States. It has also developed Gardasil for the human papillomavirus, as well as an Ebola vaccine.
The company is expected to dedicate two of its facilities to the manufacturing of Johnson & Johnson's COVID-19 vaccine. One location will focus on the last stage of production, when the vaccine substance is placed in vials and packaged for distribution. The second location will make the vaccine substance.
It is unclear when Merk will begin the manufacturing, as it is expected to take a few months to equip both facilities with the necessary equipment.
Want to get the biggest stories from PEOPLE every weekday? Subscribe to our new podcast, PEOPLE Every Day, to get the essential celebrity, entertainment and human interest news stories Monday through Friday.
The vaccine from Johnson & Johnson is the third approved to fight the ongoing COVID-19 pandemic, following the authorization of the Moderna and Pfizer vaccine. It is the first that requires only one shot and the first that can be stored at normal refrigerator temperatures for months at a time.
Around 4 million doses were distributed at sites across the U.S. since the Food and Drug Administration (FDA) authorized it for emergency use on Saturday.
RELATED: CDC Recommends One-Shot Johnson & Johnson COVID-19 Vaccine After FDA Authorizes Emergency Use
"Literally it's on trucks as we're talking," Johnson & Johnson CEO Alex Gorsky said during a Monday morning appearance on Today.
"We think literally within about the next 24-48 hours American should start receiving shots in arms," he continued. "This is the culmination of more than a year of day and night efforts on the part of our physicians, our scientists, our engineers, to have a safe, effective single shot."
The United States has already placed an order for 100 million doses of the Johnson & Johnson vaccine, although production delays have limited the number of doses that are immediately available.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from the CDC, WHO and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article