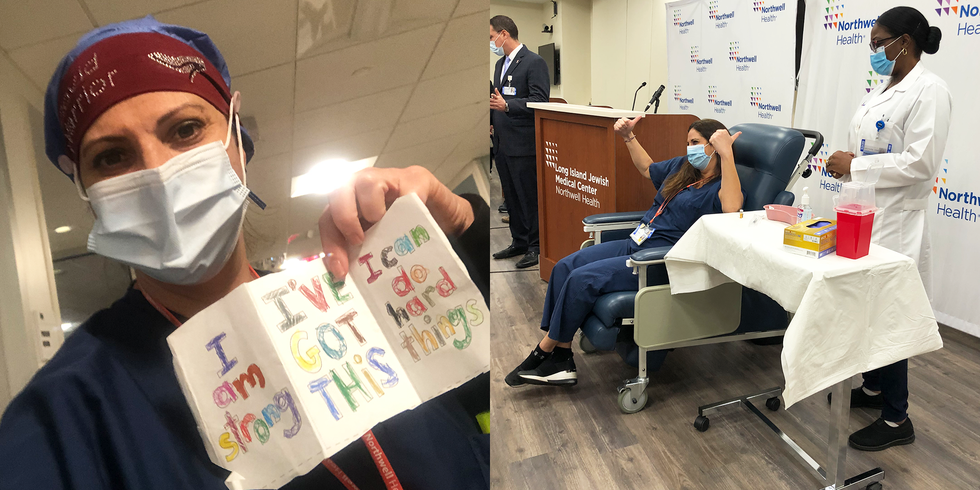
Finally, a bright spot in 2020: On Friday, December 11, the U.S. Food and Drug Administration (FDA) authorized the COVID-19 vaccine from Pfizer/BioNTech for emergency use, and by Monday morning the first shots were given. The breakthrough comes after nearly a year of scientists racing to produce a safe and effective vaccine.
The Pfizer/BioNTech vaccine requires two doses, three weeks apart, and is said to be 95 percent effective at preventing symptomatic COVID-19. The U.S. is set to distribute 2.9 million shots this week to frontline health care workers and people living in long-term care facilities.
Three other vaccines also hold promise: one from Moderna/National Institutes of Health, which is currently under FDA review and is said to have 94.1 percent efficacy, one from AstraZeneca/Oxford University (said to be 70 percent effective), and one from Johnson & Johnson (the efficacy is still unknown).
The Moderna and Astrazeneca vaccines would require two doses, four weeks apart, while the Johnson & Johnson vaccine is one-dose. This means three out of the four most promising vaccines would require a second (booster) shot, which is not uncommon for vaccines (hepatitis and HPV vaccines require multiple doses).
A quick primer: A vaccine is a substance that contains dead or weakened, disease-causing microbes. (For example, the measles vaccine contains measles microbes.) These microbes are inactive, which means they won’t make you sick—but they will stimulate your immune system to produce antibodies that will protect you from that disease in the future, according to the Centers for Disease Control and Prevention (CDC).
“Vaccines fool the body into thinking it’s being attacked without actually giving you the virus,” explains Paula Cannon, PhD, professor of microbiology and immunology at the Keck School of Medicine at the University of Southern California. “Your body scrambles to make antibodies that are tailor-made to fight that virus, and you retain those antibodies for life.” (Antibodies are proteins your immune system makes to fight infections like coronavirus.)
In the case of COVID-19, the vaccines work in different ways but they all do the same thing: They trick your body into creating antibodies that fight COVID-19. Keep reading to learn exactly how the vaccines work, when they’ll be approved, and how soon you could get vaccinated.
Is there more than one COVID-19 vaccine in the works?
Researchers are testing dozens of vaccines across the globe. According to the New York Times’ Coronavirus Vaccine Tracker, there are 59 vaccines in clinical trials on humans and approximately 86 preclinical vaccines being tested on animals. So far, the most promising vaccines are the ones being produced by Pfizer/BioNTech, Moderna/National Institutes of Health, AstraZeneca/Oxford University, and Johnson & Johnson.
What’s in the COVID-19 vaccine?
“The Pfizer/BioNTech vaccine and Moderna vaccine don’t contain the whole virus,” says Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security. “They contain genetic material from the virus, known as mRNA.” When mRNA enters your body, your cells turn it into a protein called a spike protein, which your body recognizes as foreign and forms an immune response against.
“The mRNA is like an instruction manual,” explains Cannon. “It tells your body how to make the spike protein, which is a cool trick because normally it would need to be made in a lab.” (You know those images you’ve seen of the coronavirus? Those things sticking out of the microbe are the spike protein.)
Indeed, the use of mRNA in a vaccine is a huge scientific breakthrough. “It changes the entire way we respond to infectious disease emergencies and makes vaccine development much quicker, easier, and less costly,” says Adalja. “Not only are the Pfizer and Moderna vaccines a win against COVID-19, but they’re also a win for emerging infectious diseases as a whole.”
The AstraZeneca/Oxford University vaccine and Johnson & Johnson vaccine use a different tactic to get spike protein into your body. “They also have the genetic instructions to make spike protein but instead of being written on a piece of mRNA, it’s contained within a harmless adenovirus, which normally causes the common cold,” says Cannon. The adenovirus has been weakened so it can’t infect you—it’s simply used to transport the genetic instructions for your body to make spike protein. From there, the mechanism is the same as with the Pfizer/BioNTech and Moderna vaccine: Your body sees the spike protein as foreign and creates antibodies to fight against it.
Will the COVID-19 vaccine be FDA approved?
All vaccines must be approved by the Food and Drug Administration before they can be used in the United States. FDA scientists and medical professionals carefully evaluate all the available data about the vaccine to ensure its safety and effectiveness.
On December 11, the FDA authorized the emergency use of the Pfizer/BioNTech vaccine. “Because of the urgency of the situation, the FDA has been fast-tracking the approval process,” says Jessica Malaty Rivera, MS, Science Communication lead at The COVID Tracking Project. “But that doesn’t mean the safety or efficacy has been compromised.” Nearly three million doses of the Pfizer/BioNTech vaccine are expected to be distributed this week.
Will the COVID-19 vaccine have side effects?
Like any vaccine, the COVID-19 vaccine does come with potential side effects. “Similar to the flu vaccine, the side effects are pretty mild, and the most common one is fatigue,” says Malaty Rivera.
Other potential side effects, which may last several days, include injection site soreness, muscle aches and pains, chills, joint pain, and a low-grade fever, according to the FDA. More people experience side effects after the second dose than after the first dose.
The long-term side effects of the vaccines are unknown. That said, the risk of severe side effects such as heart issues is low. “Statistically, one in a million people will have serious vaccine side effects,” says Malaty Rivera. “The general burden of the disease far outweighs the potential risks of the vaccine.”
When will the COVID-19 vaccine be distributed?
On December 14, initial doses of the Pfizer/BioNTech vaccine were rushed to injection sites across the country. The Department of Health and Human Services plans to send nearly three million doses out in the first week, and they’ve promised to provide enough vaccine for 20 million Americans by the end of the year.
Healthy adults likely won’t be able to get the vaccine until spring or summer 2021. Dr. Anthony Fauci has predicted that healthy people could walk into a drugstore and get the vaccine as early as April. If you’re wondering where you fall in the line for the vaccine, take the New York Times’ handy quiz.
Will the COVID-19 vaccine be mandatory?
In general, vaccines can’t be mandated by the federal government; however, states and cities have the authority to regulate public health and they’ve mandated vaccines in the past.
The only people who may be required to get the vaccine are healthcare workers, which isn’t unusual. Hospitals frequently make staff get the flu or hepatitis B vaccine. Schools may make the same requirement. “There are vaccine requirements for school because you’re putting yourself in a public setting where there may be other people who are medically fragile,” explains Malaty Rivera. “Also: School is something you can do on your own if you don’t agree with the protocol.”
You may have heard rumors that companies like Ticketmaster or even your own employer could make the COVID-19 mandatory, but these claims are untrue. “No vaccines are mandatory for adults,” says Adalji. “There may be some employers who want that to be the case but it will be hard to do.”
Source: Read Full Article